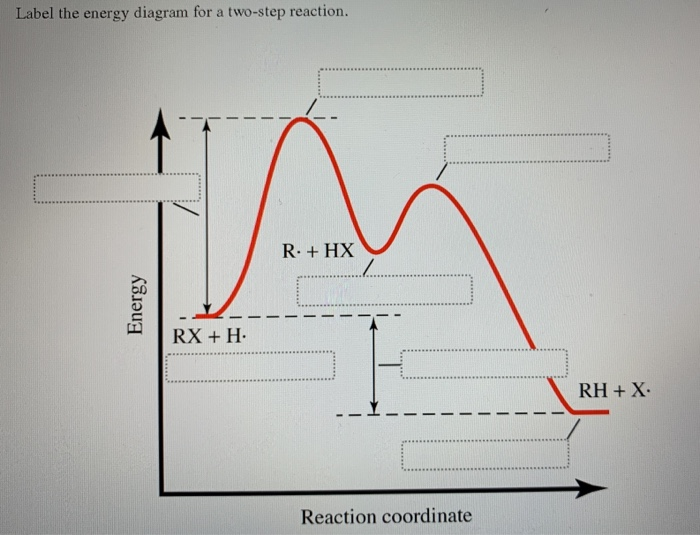
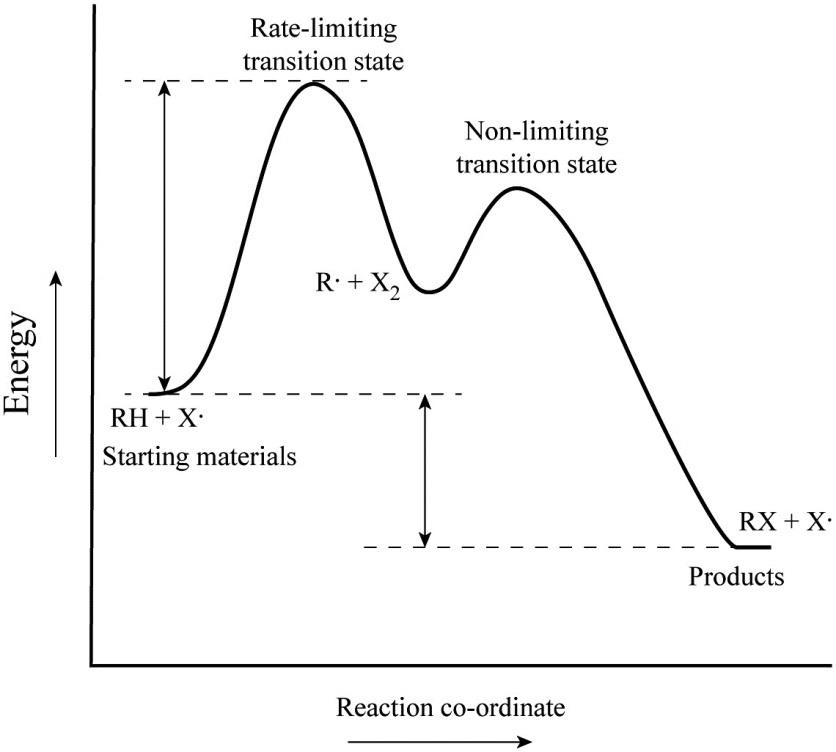
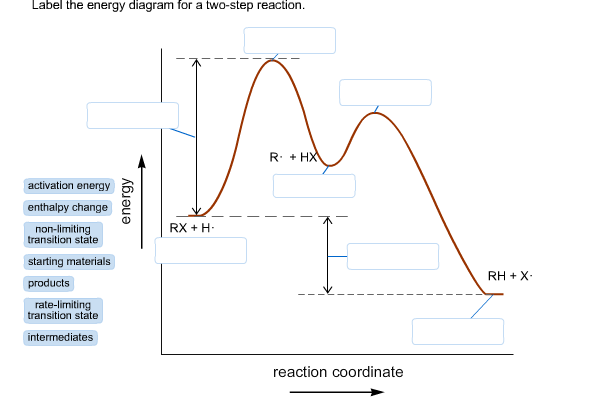
42 label the energy diagram for a two‐step reaction
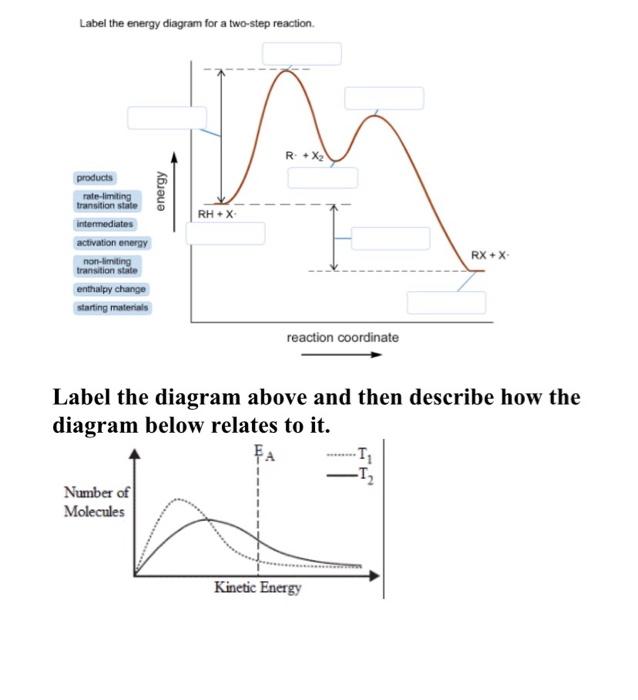
Solved: Sketch an energy diagram for a two-step reaction in which ... Sketch an energy diagram for a two-step reaction in which both steps are exergonic and in which the second step has a higher-energy transition state than the first. Label the parts of the diagram corresponding to reactant, product, intermediate, overall Δ G‡, and overall Δ G °. Step-by-step solution 89% (9 ratings) for this solution Step 1 of 4 Solved Label the energy diagram for a two-step reaction. R. Question: Label the energy diagram for a two-step reaction. R. + HX Energy RX + H . RH + X Reaction coordinate Answer Bank enthalpy change intermediates ...
Roller Coasters and Amusement Park Physics - Physics Classroom Determine the magnitude of any known forces and label on the free-body diagram. (For example, if the mass is given, then the F grav can be determined. And as another example, if there is no vertical acceleration, then it is known that the vertical forces or force components balance, allowing for the possible determination of one or more of the ...
Label the energy diagram for a two‐step reaction
Solved Label the energy diagram for a two-step reaction. Question: Label the energy diagram for a two-step reaction. activation energy non-limiting transition state D products intermediates RX + H RH +X starting ... Energy Diagram for a Two-Step Reaction Mechanism Complete Energy Diagram for Two-Step Reaction A Two-Step Reaction Mechanism The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and activation energy. The overall energy difference between the starting materials and products is delta H overall. Energy Diagrams: Describing Chemical Reactions Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DGrxn, as well as DG1* and DG2* for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk.
Label the energy diagram for a two‐step reaction. Question paper (A-level) : Paper 2 Organic and physical ... - AQA -diaminohexane by the reaction of 1,6-dibromohexane with an excess of ammonia. [2 marks] 0 1 . 2 Complete the mechanism for the reaction of ammonia with 6. form 1,6-bromohexylamine to -diaminohexane. Suggest the structure of a cyclic secondary amine that can be formed as a by-product in this reaction. [4 marks] Mechanism Cyclic secondary amine Energy Diagrams of Two Step Reactions - YouTube Watch Complete videos @ Organic Chemistry 1 Draw an energy diagram for each reaction. Label the axes, the starting ... Draw an energy diagram for each reaction. Label the axes, the starting material, product, transition state, Δ Δ H , and Ea. a. a concerted reaction with Δ Δ H = -80 kJ/mol and Ea = 16 kJ/mol b. a... Cold fusion - Wikipedia Cold fusion is a hypothesized type of nuclear reaction that would occur at, or near, room temperature.It would contrast starkly with the "hot" fusion that is known to take place naturally within stars and artificially in hydrogen bombs and prototype fusion reactors under immense pressure and at temperatures of millions of degrees, and be distinguished from muon-catalyzed fusion.
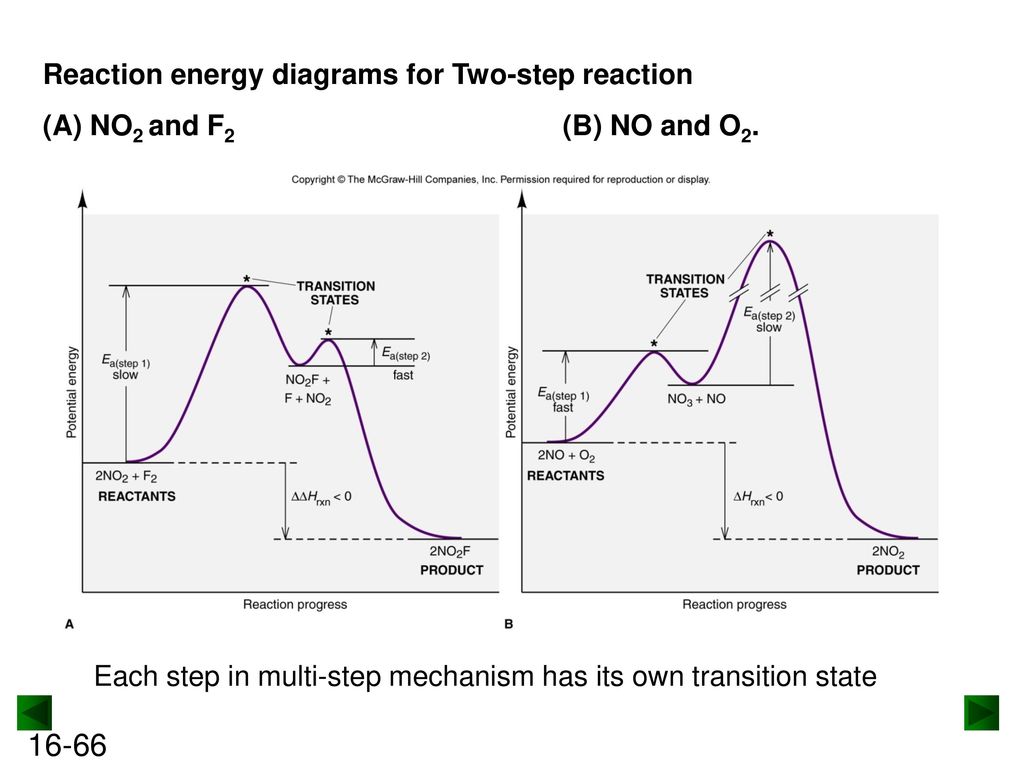
Label the energy diagram for a two-step reaction. - Chegg Question: Label the energy diagram for a two-step reaction. This problem has been solved! See the answer ... Solved Label the energy diagram for a two-step reaction. R Question: Label the energy diagram for a two-step reaction. R: + HX Energy RX + H RH + X Reaction coordinate Answer Bank products enthalpy change activation ... Energy Diagrams of Reactions | Fiveable To find the activation energy, you should be looking for two numbers: the potential energy of the reactants and the energy of the activated complex (the maximum point). (energy of activation complex) - (PEreactants) (100 kJ) - (40 kJ) = 60 kJ In other words, it takes 60 kJ of energy to complete the reaction. Labeling an Energy Diagram Diagram | Quizlet Labeling an Energy Diagram STUDY Learn Flashcards Write Spell Test PLAY Match Gravity Created by Corey_WilliamsonPLUS Terms in this set (9) Reactants Starting ingredients for Forward reaction Forward Activation Energy (Ea) Energy required to break the bonds between atoms for the FORWARD reaction Enthalpy (∆H)
Solved: Draw an energy diagram for a two-step reaction with Keq ... Problem 29E Draw an energy diagram for a two-step reaction with Keq > 1. Label the overall Δ G °, transition states, and intermediate. Is Δ G ° positive or negative? Step-by-step solution 95% (19 ratings) for this solution Step 1 of 3 Gibb's free-energy is the energy difference between the reactants and the products. Solved Label the energy diagram for a two-step reaction. R Question: Label the energy diagram for a two-step reaction. R: + HX Energy RX + H RH + X Reaction coordinate Reaction coordinate Answer Bank intermediates ... Draw an energy diagram for a two-step reaction, - Quizlet Find step-by-step Chemistry solutions and your answer to the following textbook question: Draw an energy diagram for a two-step reaction, $$ A \rightarrow B \rightarrow C, $$ where the relative energy of these compounds is C < A < B, and the conversion of $$ B \rightarrow C $$ is rate-determining.. Krebs cycle / Citric acid cycle / TCA Cycle with steps and ... Mar 10, 2022 · This reaction is a reversible reaction catalyzed by the enzyme (aconitase). This reaction takes place by a two-step process where the first step involves dehydration of citrate to cis-aconitase, followed by the second step involving rehydration of cis-aconitase into isocitrate. Step 3: Oxidative decarboxylations of isocitrate
Solved Label the energy diagram for a two-step reaction. R. Question: Label the energy diagram for a two-step reaction. R. + HX M Energy RX + H RH + X Answer Bank intermediates enthalpy change products starting materials ...
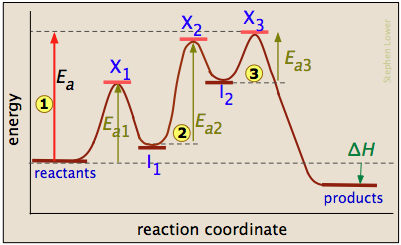
Answered: Draw a potential energy diagram given… | bartleby draw a potential energy diagram given the following information. - the reaction proceeds in three steps - the activation energy of step 2 is double that of step 1. - the activation energy of step 3 is double that of step 2 - the reaction is endothermic label all axes, the rate limiting step, the location of any intermediates and transition …
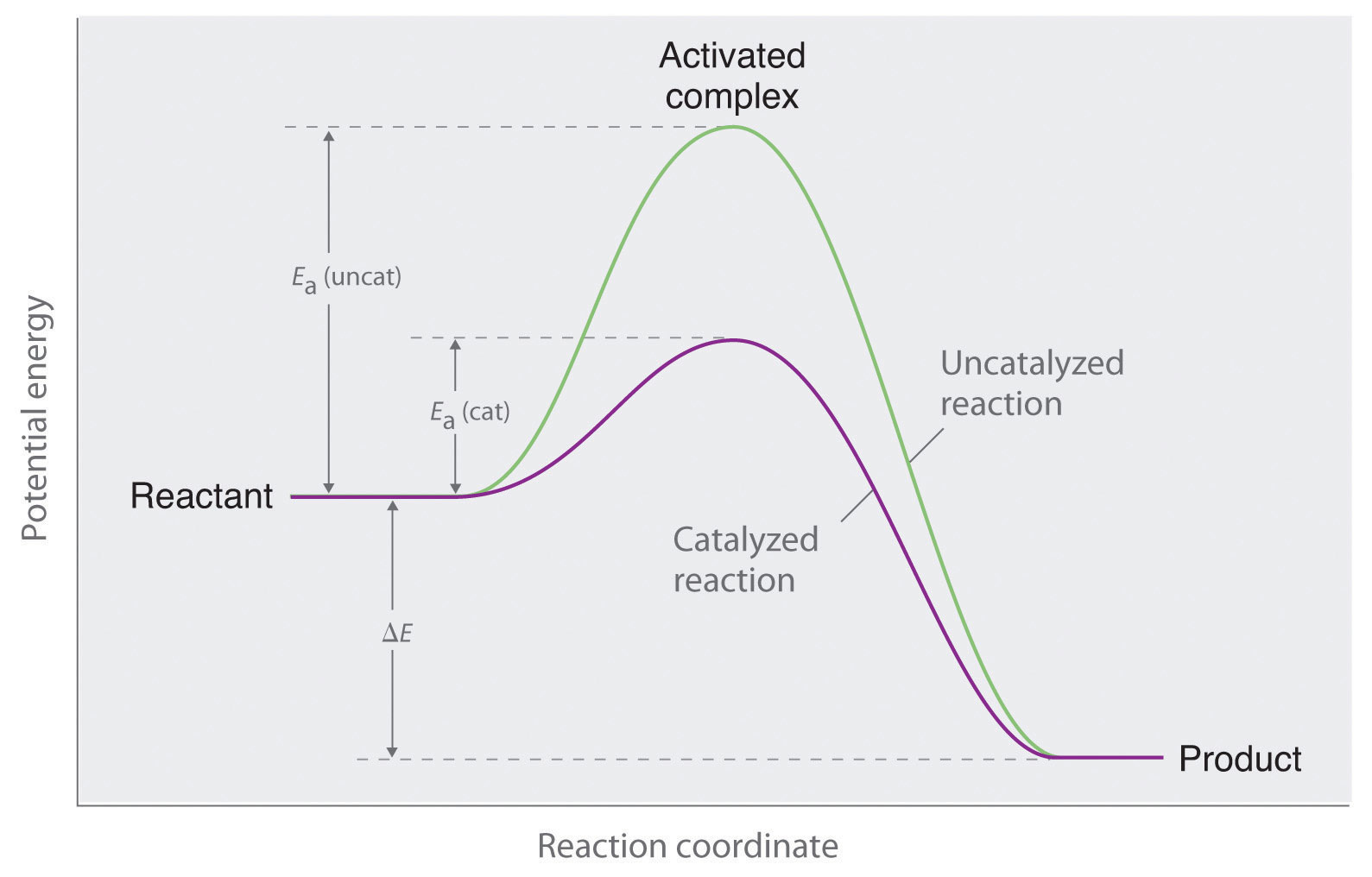
Potential Energy Diagrams & Activation Energy - Online Math Learning How to draw and label PE diagrams for exothermic and endothermic reactions and what effect adding a catalyst or inhibitor has on the diagram. Chemistry Tutorial 9.02b: Potential Energy Diagrams. Watch on. Activation energy. Activation energies at exothermic and endothermic reactions.
How to draw the potential energy diagram for this reaction? 2. Identify the sign of the enthalpy change, ΔH, along with its value. The decrease in chemical potential energy should be the same as the amount of thermal energy released. That is. Ereactants − Eproducts = 2219.9lkJ⋅ mol−1. However, since. ΔH = Eproducts −Ereactants , ΔH = −2219.9lkJ⋅ mol−1. Note that by convention, the ...
Optical Metasurfaces for Energy Conversion | Chemical Reviews Nanostructured surfaces with designed optical functionalities, such as metasurfaces, allow efficient harvesting of light at the nanoscale, enhancing light–matter interactions for a wide variety of material combinations. Exploiting light-driven matter excitations in these artificial materials opens up a new dimension in the conversion and management of energy at the nanoscale. In this review ...
Answered: Kinetics: Draw a Potential energy… | bartleby Q: Draw an energy diagram for each reaction. Label the axes, the starting material, product, transition… A: a. This is a one step reaction A gives B. This reaction is exothermic since B is lower than A.
Chapter 19, Problem 65P | bartleby A firewalker runs across a bed of hot coals without sustaining burns. Calculate the heat transferred by conduction into the sole of one foot of a firewalker given that the bottom of the foot is a 3.00-mm-thick callus with a conductivity at the low end of the range for wood and its density is 300 kg/m3.
SOLVED:Draw a reaction coordinate diagram for a two-step ... - Numerade here for the reaction. Coordinate diagram. Put two step verification in which the first step, Andrea Janek, the second step is extra, Janek. And the order reaction is and a sonic labels are re intense. Products, intermediates and chronic transition is start stairs.
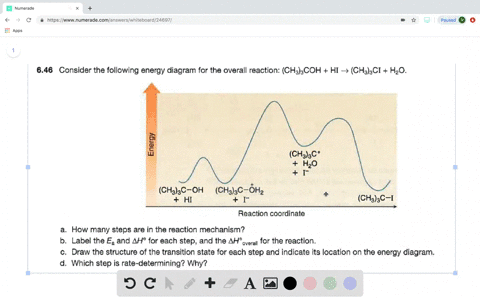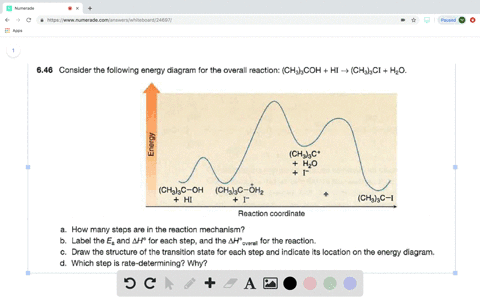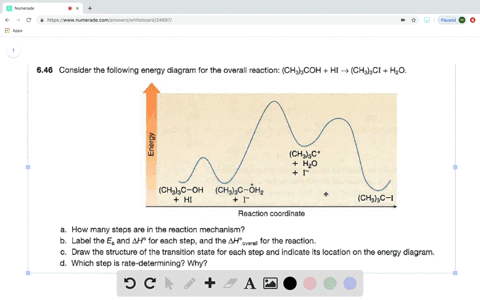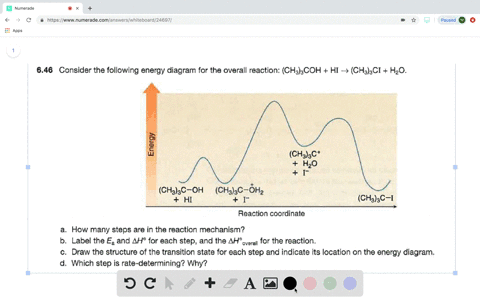
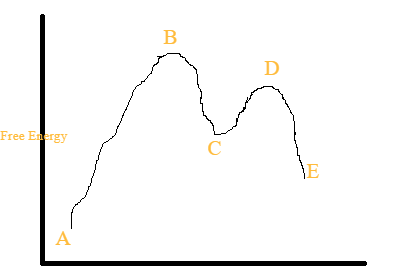
Answered: Consider the following energy diagram… | bartleby How many steps are present in the reaction mechanism? d. Label each step of the mechanism as endothermic or exothermic. e. Label the overall reaction as endothermic or exothermic. B G Reaction coordinate Energy ... Sketch an energy diagram for a two-step reaction in which both steps are exergonic and in which the ...
Label the energy diagram for a two-step reaction. - Chegg Answer to Solved Label the energy diagram for a two-step reaction.
Label the energy diagram for a two-step reaction. R - Chegg Question: Label the energy diagram for a two-step reaction. R: + HX ТЕУ Answer Bank rate-limiting transition state starting materials products non-limiting ...
Engineering Chemistry by Jain & Jain - Academia.edu Enter the email address you signed up with and we'll email you a reset link.
Mechanisms and Potential Energy Diagrams - Course Hero A potential energy diagram for a two-step reaction is shown and labeled. Practice View the section on two-step reactions at the site below and then do the self-test (both buttons are at the top of the slide). Don't worry about - just consider it an indication of activation energy as is in the diagram above.
Potential Energy Diagrams Answer-->. KNO 3 NH 4 Cl NH 4 NO 3 NaCl. 6/04. 21 A catalyst increases the rate of a chemical reaction by. (1) lowering the activation energy of the reaction (2) lowering the potential energy of the products. (3) raising the temperature of the reactants (4) raising the concentration of the reactants. Answer-->.
PDF Discussion Worksheet #6 Substitution Reactions Sn2 is a one step, 2 arrow mechanism with alkyl halides. Sn1 is a two step, 2 arrow mechanism (often followed by a deprotonation step) with alkyl halides. Problem 1: Label each as Sn1 or Sn2. Provide arrow mechanisms for each reaction, and draw an energy diagram assuming an overall exothermic process.
Answered: Draw a reaction energy diagram for a… | bartleby Science Chemistry Q&A Library Draw a reaction energy diagram for a two-step exothermic reaction whose second step is faster than the first step. Label the intermediate, transition states, ΔH, and activation energies.
Question #62891 | Socratic The figure above represents the reaction profile of a two step, exothermic reaction. The y-axis represents the potential energy of the reaction species, and the x-axis represents the progress of the reaction. The reaction is exothermic because the energies of the products are lower than those of the reactants. The reactants are represented by the horizontal line at the far left of the graph ...
How can I draw activation energy in a diagram? | Socratic 2. Draw and label two short horizontal lines to mark the energies of the reactants and products. 3. Draw the energy level diagram. There must be a hump in the curve to represent the energy level of the activated complex. 4. Draw and label the activation energy. Draw a horizontal line from the highest part of the curve towards the vertical axis.
Solved Label the energy diagram for a two-step reaction. - Chegg Question: Label the energy diagram for a two-step reaction. enthalpy change transition state starting materials RX+H products rate-limiting transition state intermediates activation energy reaction coordinate This problem has been solved! See the answer Show transcribed image text Expert Answer 100% (54 ratings)
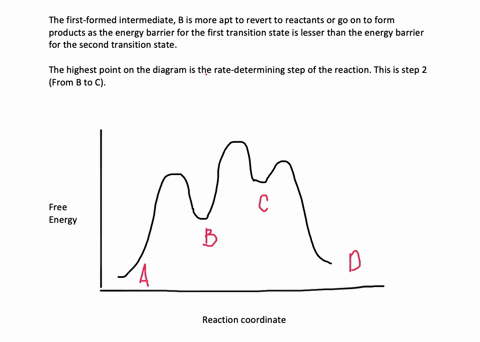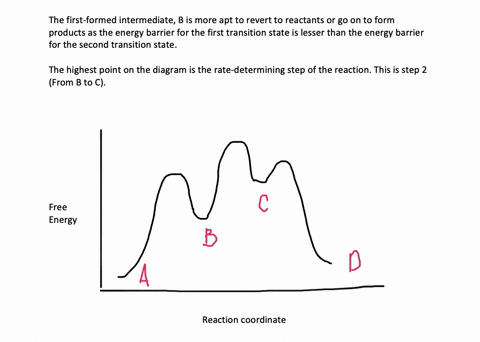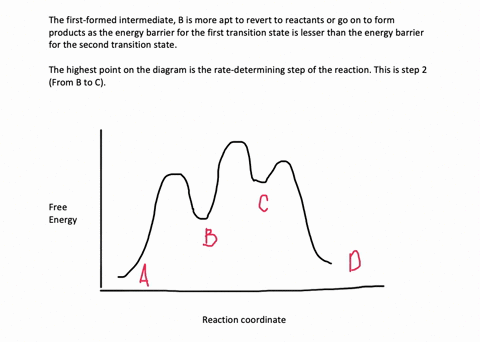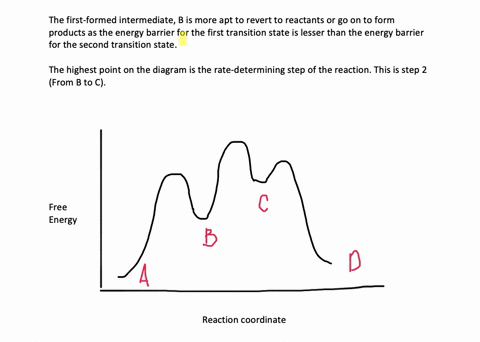
Multistep Reactions - Softschools.com The energy diagram of a two-step reaction is shown below. In the above reaction, a reactant goes through one elementary step with a lower activation energy (transition state 1) to form the intermediate. The intermediate then goes through a second step (transition state 2) with the highest energy barrier to form the product.
How does the energy level diagram show this reaction is exothermic? Label ΔH as positive or negative. Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases.
Energy Diagrams: Describing Chemical Reactions Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DGrxn, as well as DG1* and DG2* for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk.
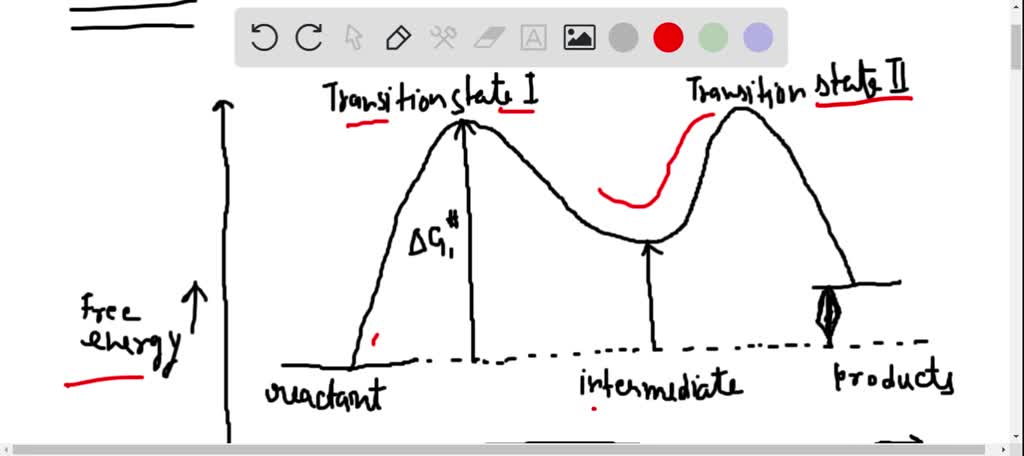
Energy Diagram for a Two-Step Reaction Mechanism Complete Energy Diagram for Two-Step Reaction A Two-Step Reaction Mechanism The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and activation energy. The overall energy difference between the starting materials and products is delta H overall.
Solved Label the energy diagram for a two-step reaction. Question: Label the energy diagram for a two-step reaction. activation energy non-limiting transition state D products intermediates RX + H RH +X starting ...

















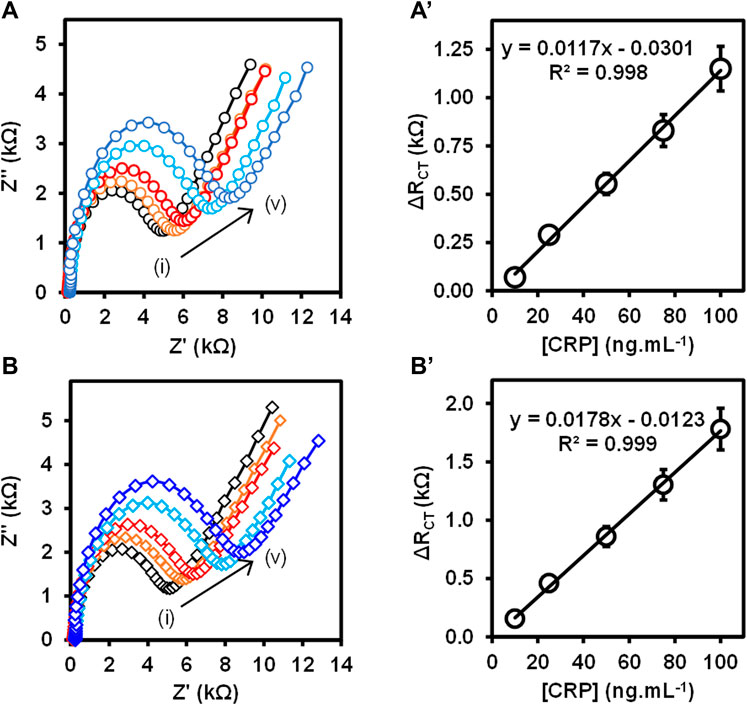
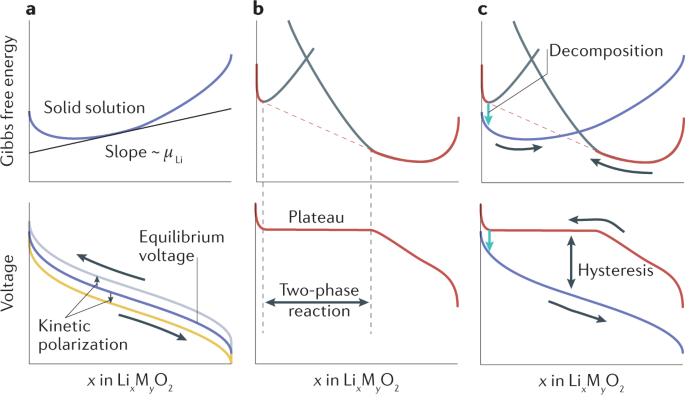





Post a Comment for "42 label the energy diagram for a two‐step reaction"