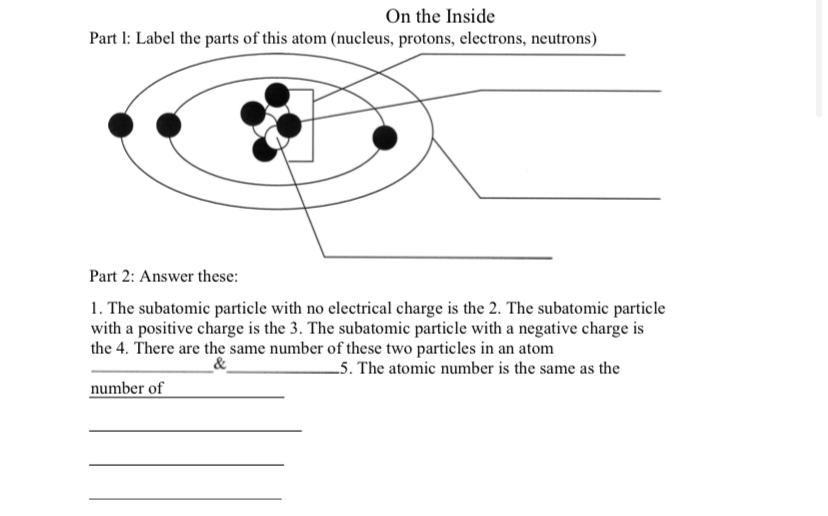
41 atom and label its parts
Structure of the atom - Atomic structure - AQA - BBC Bitesize Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The numbers of subatomic particles in an atom can be calculated from its atomic number and mass... What Are The 5 Parts Of An Atom. Atom is divided into. Nucleus. Electron shell. Nucleus features following. Protons. Neutrons. electrons.
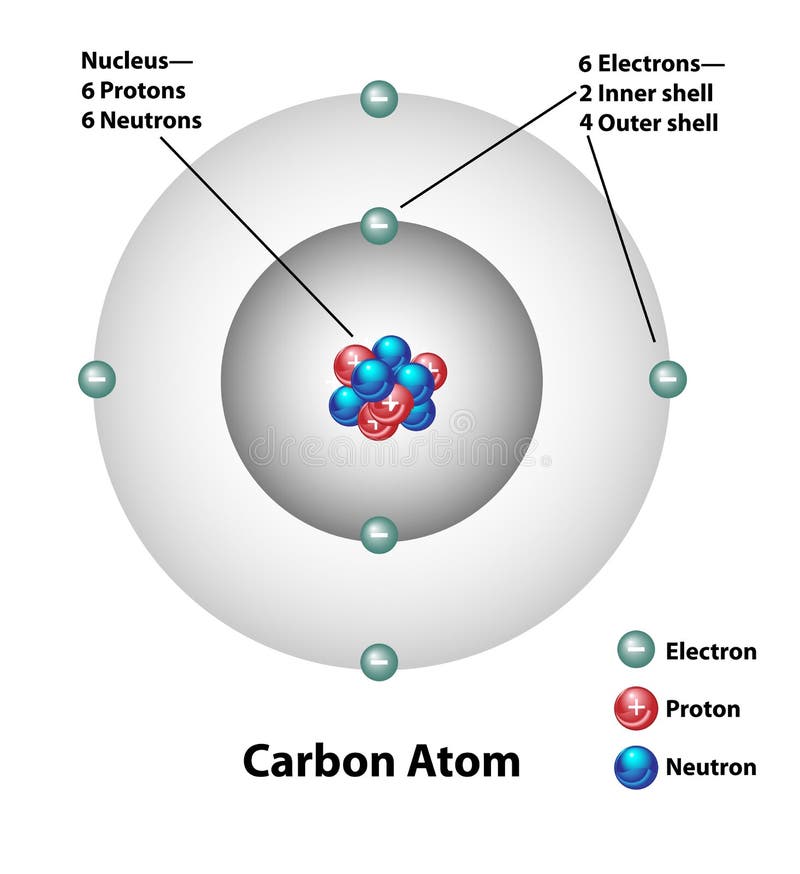
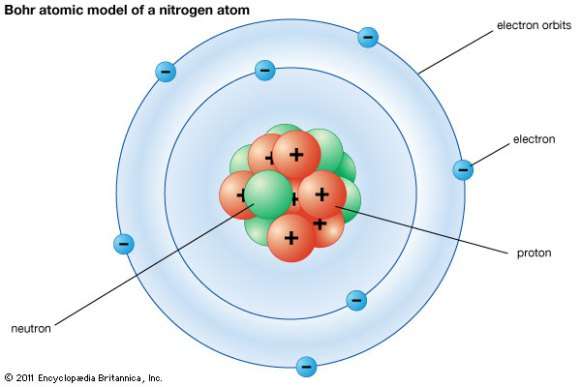
Chem4Kids.com: Atoms: Structure Every element is unique and has an atomic number. That number tells you the number of protons in every atom of the element. The atomic number is also called the proton number. Charges of Atoms You can see that each part of the atom is labeled with a "+", "-", or a "0." Those symbols refer to the charge of the particle.
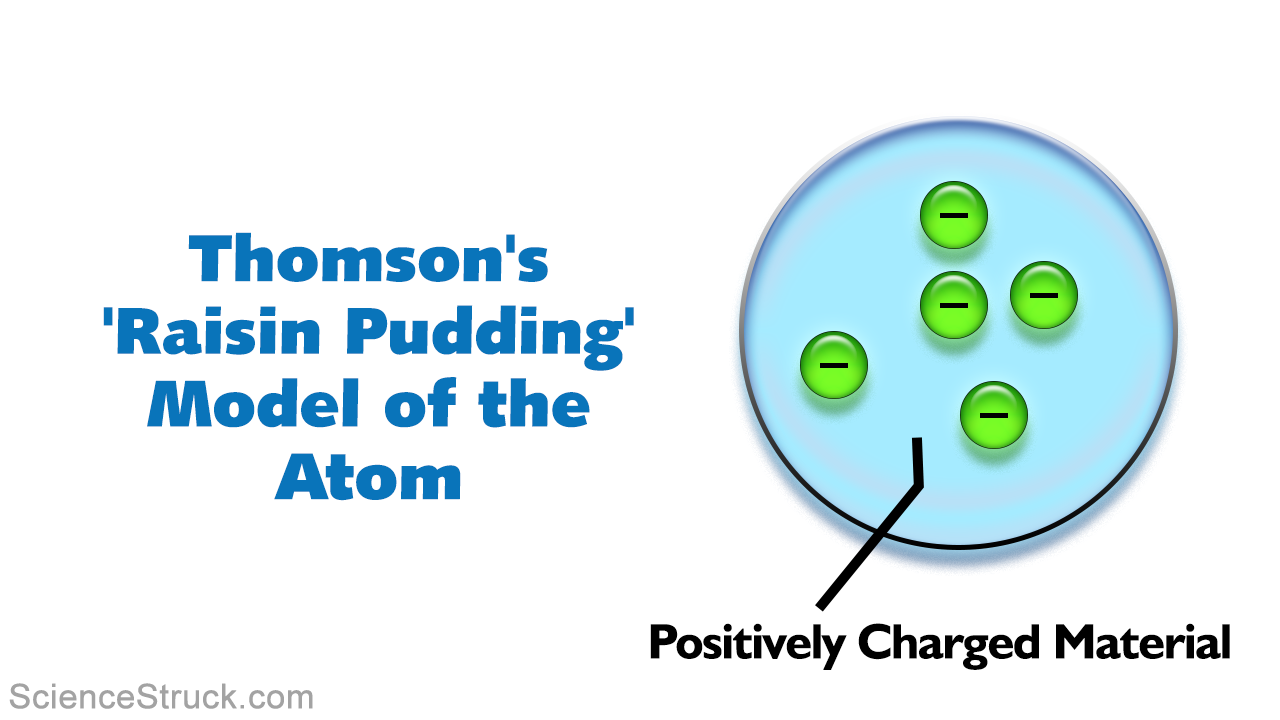
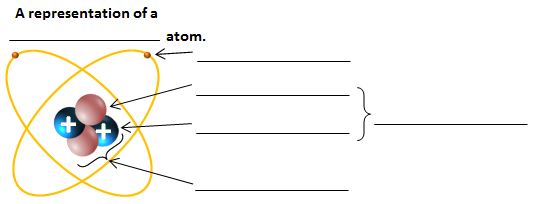
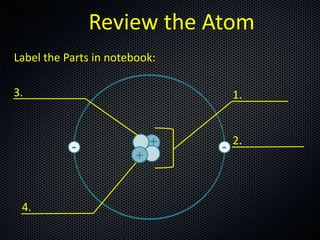
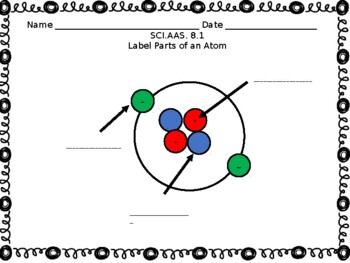
Atom and label its parts
Cell Size and Scale - University of Utah The label on the nucleotide is not quite accurate. Adenine refers to a portion of the molecule, the nitrogenous base. It would be more accurate to label the nucleotide deoxyadenosine monophosphate, as it includes the sugar deoxyribose and a phosphate group in addition to the nitrogenous base. Atom Diagram - Universe Today An atom consists of three main parts: protons, neutrons, and electrons. Protons have a positive electrical charge. Neutrons have no electrical charge. Electrons have a negative electrical charge.... Label the Atom Diagram | Quizlet Center of the atom. Contains the protons and neutrons Electron Negative particles in the electron cloud. Discovered by JJ Thomson Neutron Particle with no charge in the nucleus of an atom. Discovered by Chadwick Electron Cloud Area outside the nucleus where electrons are located Proton Positive particles found in the nucleus of an atom.
Atom and label its parts. highlight.js demo Arduino /* Blink Turns on an LED on for one second, then off for one second, repeatedly. This example code is in the public domain. */ // Pin 13 has an LED connected on most Arduino boards. // give it a name: int led = 13; // the setup routine runs once when you press reset: void setup() { // initialize the digital pin as an output. Structure of the atom - The atom - BBC Bitesize Atoms contain three sub-atomic particles called protons, neutrons and electrons. The protons and neutrons are found in the nucleus at the centre of the atom. The nucleus is very much smaller than... Activity 3.The AT M Familyobjectives:* Describe the stem and its ... Drawan atau and label its parts 。 Direction: mt 1. Read the basie information about the atom and answer the questions The term stom" came from a Greek word "atomom* meaning adivinible". Today, an atom is defined as the smallest particle that makes up matter, Far decades, scientists have been gathering evidence about its structure. Their Introduction to Structure of Atom - Toppr-guides Structure of Atom The structure of atom consists of two parts: an atomic nucleus extra nucleus part The tiny atomic nucleus is the center of an atom. It constitutes positively charged particles "protons" and uncharged particles " neutrons ." On the other hand, the extra nucleus part is a much larger region.
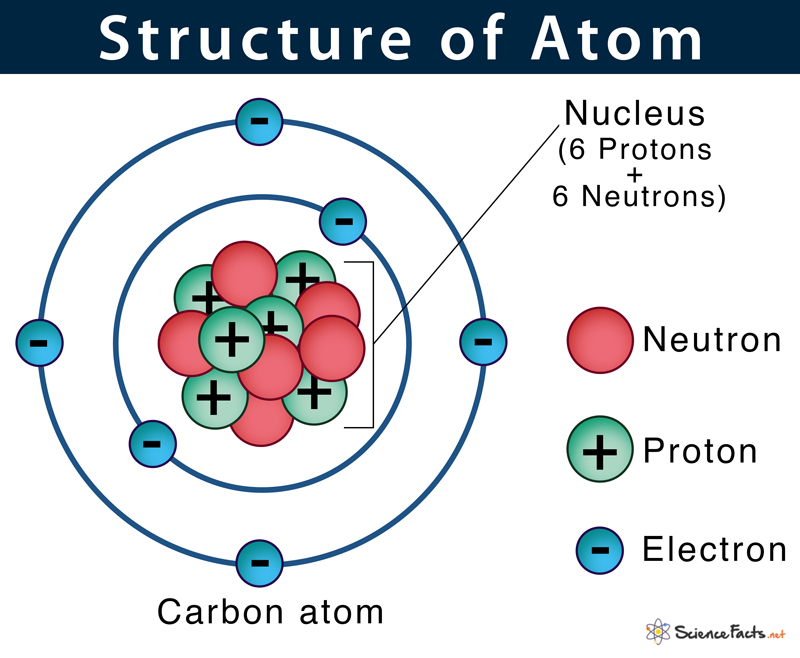
Build an Atom - Atoms | Atomic Structure | Isotope Symbols ... Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! 3 Parts of a Nucleotide and How They Are Connected - ThoughtCo The phosphorus atom is the central atom. One atom of oxygen is connected to the 5-carbon in the sugar and to the phosphorus atom. When phosphate groups link together to form chains, as in ATP (adenosine triphosphate), the link looks like O-P-O-P-O-P-O, with two additional oxygen atoms attached to each phosphorus, one on either side of the atom. Atomic Structure - Electrons, Protons, Neutrons and Atomic Models - BYJUS Primarily, the atomic structure of matter is made up of protons, electrons and neutrons. The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom. The atomic number of an element describes the total number of protons in its nucleus. Atom - Wikipedia An atom is the smallest unit of ordinary matter that forms a chemical element. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics, as if they were tennis balls for example, is not possible due to quantum effects.
Terms that describe the parts of an atom Flashcards | Quizlet Terms that describe the part of an atom Learn with flashcards, games, and more — for free. Home. Subjects. Expert solutions. Create. ... Only $35.99/year. Science. Physics; Terms that describe the parts of an atom. Flashcards. Learn. Test. Match. Flashcards. Learn. Test. Match. Created by. lizzicess. Terms that describe the part of an atom ... Model a carbon atom, and label its parts. Then use a label to point out ... Correct answers: 1 question: Model a carbon atom, and label its parts. Then use a label to point out and briefly explain why carbon can form a variety of organic compounds. What are the parts of an atom? - Phys.org Our current model of the atom can be broken down into three constituents parts - protons, neutron, and electrons. Each of these parts has an associated charge, with protons carrying a positive... Structure of an Atom and its parts - Chemistry for Kids | Mocomi What are atoms made of? An atom is made of three parts - protons, neutrons and electrons. Each of these parts has an associated charge. The protons carry a positive charge, electrons have a negative charge and neutron possess no charge. Protons and neutrons make up the nucleus of the atom and electrons orbit the nucleus at different energy levels.
ATOMUSDT: 13.151 | Binance Spot Trading Targeting cookies may be set through our site by ourselves and our advertising partners. First parties and third parties will use them to build a profile of your interests based on the browsing information they collect from you, which includes uniquely identifying your browser and terminal equipment.
The Structure of an Atom: Parts, Diagram, Examples - Embibe Exams An atom consists of three elementary subatomic particles, i.e., protons, electrons, and neutrons. 2. Protons and neutrons reside in the nucleus and are together called nucleons. 3. The nucleus is positively charged since the proton is positively charged and the neutron is neutral. 4. The electrons are negatively charged. 5.
Intel® Product Specifications Intel® product specifications, features and compatibility quick reference guide and code name decoder. Compare products including processors, desktop boards, server products and networking products.
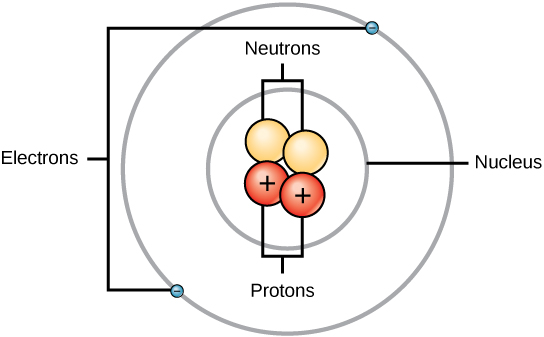
Basic Model of the Atom - Atomic Theory - ThoughtCo The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). Protons and neutrons form the atomic nucleus. Electrons are attracted to the protons in the nucleus, but are moving so quickly they fall toward it (orbit) rather than stick to protons.
The Atom 7″ Figure with The Flash Comic (Page Punchers) As the new Atom, Ryan fights to protect people just as his mentor did and serves as a member of the Justice League of America. Product Features: Incredibly detailed 7” scale figures based off the DC Multiverse; Designed with Ultra Articulation with up to 22 moving parts for full range of posing and play
atom | Definition, Structure, History, Examples, Diagram, & Facts It is composed of protons, which have a positive charge, and neutrons, which have no charge. Protons, neutrons, and the electrons surrounding them are long-lived particles present in all ordinary, naturally occurring atoms. Other subatomic particles may be found in association with these three types of particles.
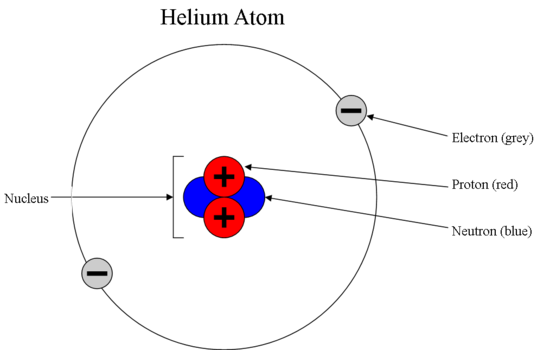
5 Different Atomic Models- Theories, Diagram & Structure of Atom The word atom came from the Greek word Atomos which means indivisible. Atoms are made of electrons, Protons, and Neutrons. Protons and Neutrons reside in the nucleus of the atom and electrons orbit the nucleus. Atoms always have an equal number of protons and electrons and the number of neutrons and protons is usually the same as well.
What is an atom? Facts about the building blocks of the universe Atoms are made up of a nucleus, protons and electrons. Atoms consist of a nucleus made of protons and neutrons orbited by electrons. (Image credit: Rost-9D via Getty Images) Atoms are the basic ...
Basic Parts of the Atom - Protons, Neutrons, Electrons, Nucleus The Beautiful pictures of the atom in this video come from Jefferson Lab @ . They are here on youtube too @ ...
The Structure of an Atom Explained With a Labeled Diagram They could explain that an atom is made up of electrons, neutrons, and protons. The center of an atom is the nucleus that contain protons and neutrons. This makes the nucleus positively charged. The electrons are present on different shells or orbits that revolve around the nucleus. This helps in determining the size of an atom.
What are the parts of an atom? What does each do? Definitions and ... Atom is made up of 4 parts, nucleus, protons, electrons and neutrons. Explanation: Protons are positively charged particles and they determine what element the atom is Neutrons mainly stabilize the atom and have the same atomic mass as protons,also located near the proton
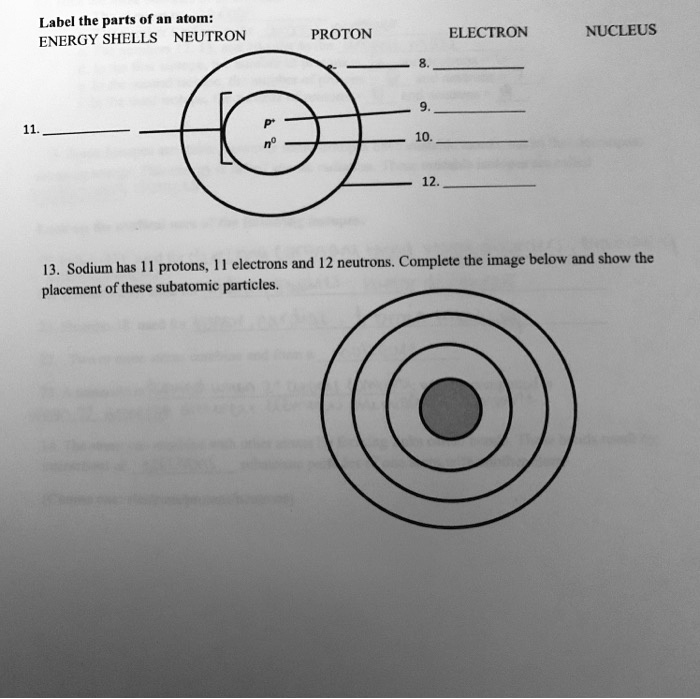
Labeled Parts of an Atom Diagram | Atom diagram, Atom, Worksheets Description Students will label parts of an atom, use the periodic table to gather information about atoms, and sketch Bohr diagrams. Students will also practice calculations to determine the amount of neutrons for any particular atom. M Mary Clark chemistry Chemistry Revision 11th Chemistry Study Chemistry Chemistry Worksheets
The Feynman Lectures on Physics Vol. III Ch. 12: The ... The electron can have its spin either “up” or “down,” and the proton can also have its spin either “up” or “down.” There are, therefore, four possible spin states for every dynamical condition of the atom. That is, when people say “the ground state” of hydrogen, they really mean the “four ground states,” and not just the ...
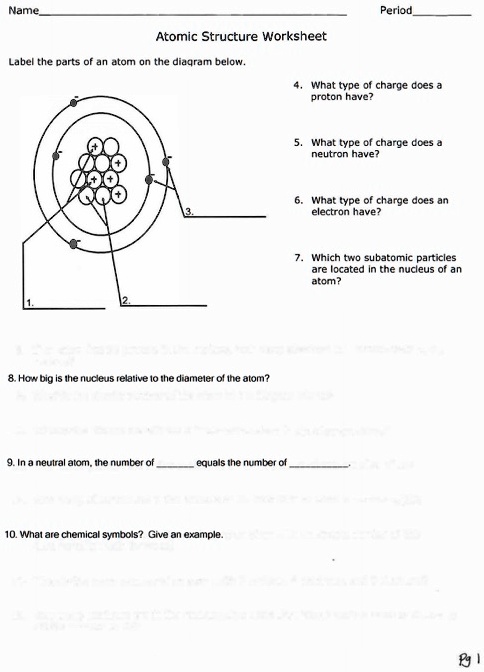
PS.docx - I. Draw an atom and label its parts. (10pts) II.... I. Draw an atom and label its parts. (10pts) II. Answer the statement briefly (10pts) 1. Given that an atom is neutral, its atomic mass (A) is the total number of its protons and neutrons and its atomic number (Z) is the total number of its protons. How will you find out the total number of electrons? The number of electrons is equal to the number of protons.
What Are The Parts Of An Atom? - Universe Today Our current model of the atom can be broken down into three constituents parts - protons, neutron, and electrons. Each of these parts has an associated charge, with protons carrying a positive...
Atoms - What are they? What's inside them? - Explain that Stuff What are the parts of an atom? Most atoms have three different subatomic particles inside them: protons, neutrons, and electrons.The protons and neutrons are packed together into the center of the atom (which is called the nucleus) and the electrons, which are very much smaller, whizz around the outside.When people draw pictures of atoms, they show the electrons like satellites spinning round ...
Part A: Atomic Structure - DocsLib Part A: Atomic Structure 1. Draw five protons in the nucleus of the atom. Label them with their charge. 2. Draw six neutrons in the nucleus of the atom. 3. Draw two electrons in the first energy level and label them with their charge. 4. Draw three electrons in the second energy level and label them with their charge. 5.
Label the Atom Diagram | Quizlet Center of the atom. Contains the protons and neutrons Electron Negative particles in the electron cloud. Discovered by JJ Thomson Neutron Particle with no charge in the nucleus of an atom. Discovered by Chadwick Electron Cloud Area outside the nucleus where electrons are located Proton Positive particles found in the nucleus of an atom.
Atom Diagram - Universe Today An atom consists of three main parts: protons, neutrons, and electrons. Protons have a positive electrical charge. Neutrons have no electrical charge. Electrons have a negative electrical charge....
Cell Size and Scale - University of Utah The label on the nucleotide is not quite accurate. Adenine refers to a portion of the molecule, the nitrogenous base. It would be more accurate to label the nucleotide deoxyadenosine monophosphate, as it includes the sugar deoxyribose and a phosphate group in addition to the nitrogenous base.
























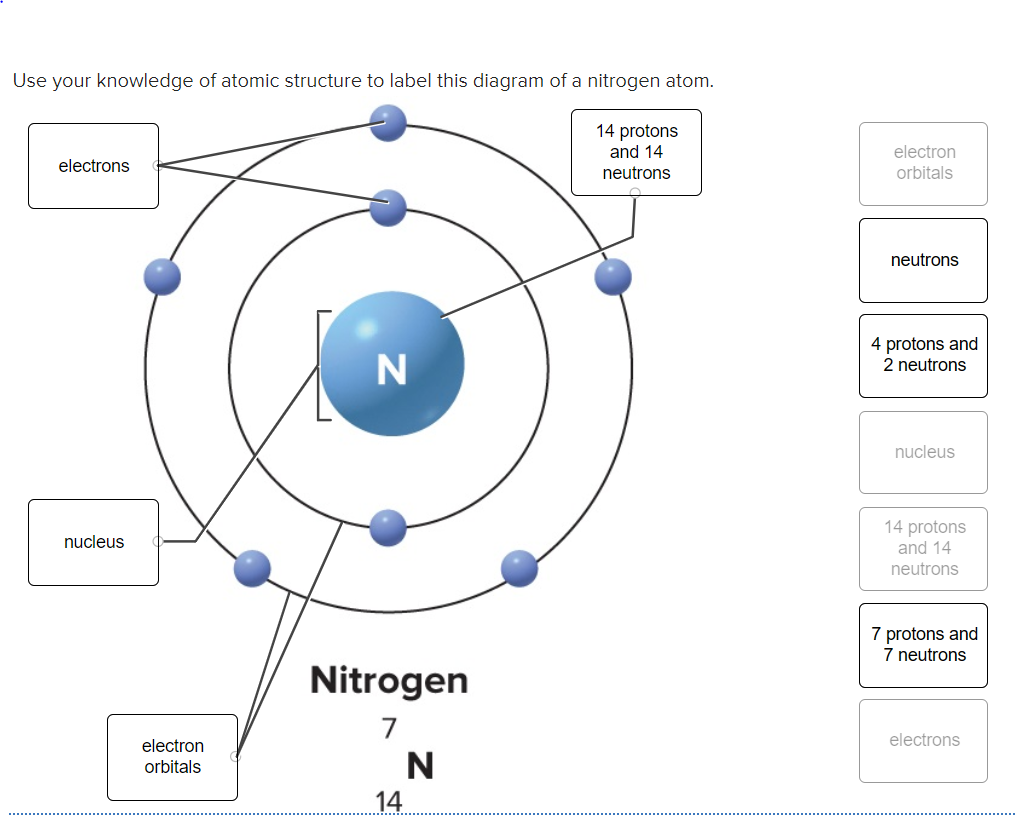
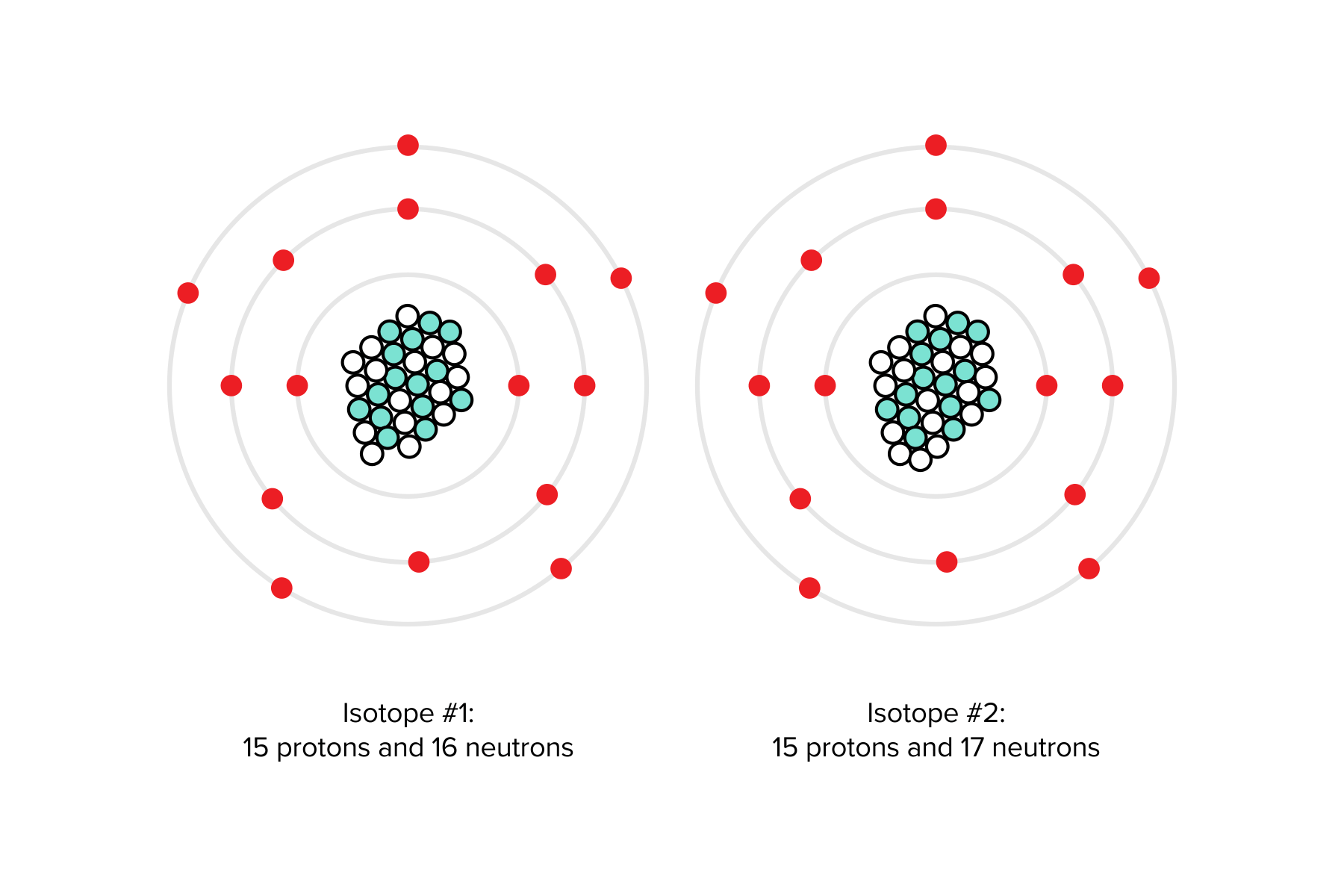
Post a Comment for "41 atom and label its parts"